Key Safety Considerations for Power Supplies Used in Home Healthcare Applications
March 7, 2023 by Ron Stull - 7 Minute Read
The Internet of Medical Things
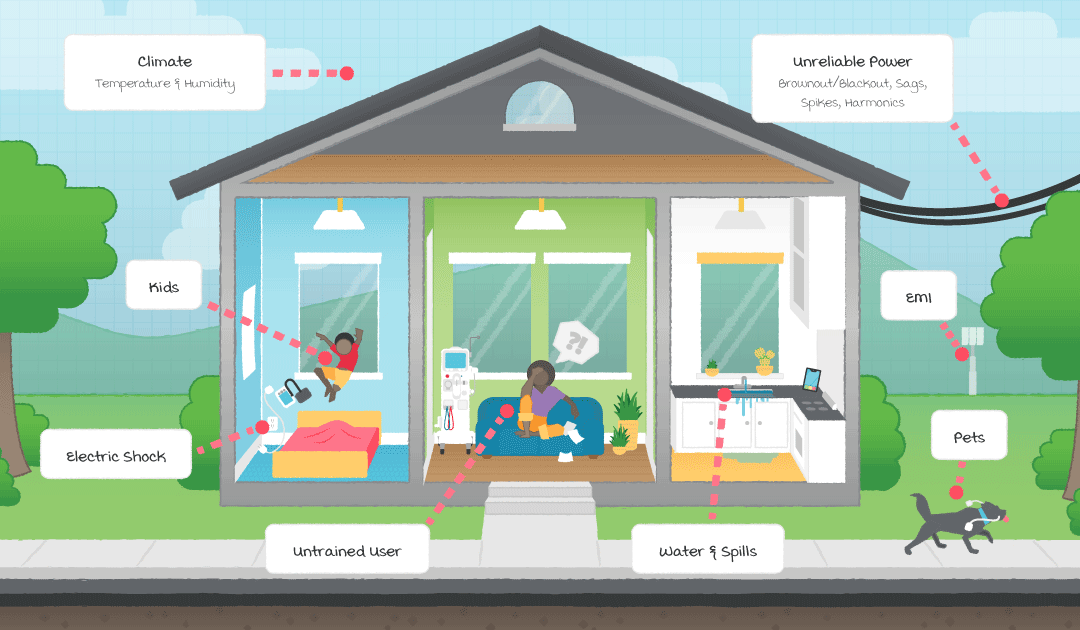
Home healthcare is not new, but the growth of the Internet of Things (IoT) has led to the so-called Internet of Medical Things (IoMT) and the development of a wide range of new medical devices that are suitable for home use. These devices can reduce healthcare cost, provide real-time monitoring of vital signs and other health indicators, and are generally more convenient for the patient. However, for all these benefits, the home environment introduces several risks that are not present in a clinical environment. These include:
- Untrained users of medical equipment
- Inconsistent power quality and infrastructure
- Uncontrolled environment
IEC 60601-1-11 Home Healthcare Collateral Standard
To address these concerns, the International Electrotechnical Commission (IEC) developed IEC 60601-1-11, a collateral standard to IEC 60601, that defines the general safety requirements and performance of medical electrical equipment used in a home healthcare environment. This standard applies additional requirements to equipment used in the home healthcare environment to account for the differences between the home and clinical environment, including untrained users and a less controlled environment.
As with many applications, the power supply is a critical component when it comes to ensuring safety, and several of the additional requirements placed on home medical equipment directly impact the choice of power supply. In this blog, we describe some of the key considerations to make when selecting a power supply for the home healthcare environment.
Home Healthcare Devices Require a Wider Input Voltage Range
Compared to the clinical environment, homes may be more susceptible to large drops in the mains voltage. If the voltage drops below the input voltage range of the power supply, the power supply may cease operation and could even lead to failure. This is not acceptable for medical equipment. While the base standard assumes a mains voltage range of 90-110% of the nominal voltage, the home healthcare standard applies a range of 85-110% for non-life supporting equipment and 80-110% for life-supporting equipment.
For a universal input range (i.e., 100-240 Vac nominal), this results in the following input voltage ranges:
- Non-life supporting equipment: 85 Vac to 264 Vac
- Life-supporting equipment: 80 Vac to 264 Vac
Challenges with Protective Earth Connection in Homes
Across the world, the quality of the local power infrastructure and requirements can vary greatly from home to home and location to location. The result is that not all power outlets may have a protective earth connection or, if it exists, it cannot be guaranteed to be installed properly. Because of this, medical equipment for the home healthcare environment that is not permanently installed must be Class II or internally powered, must not contain a functional earth terminal, and applied parts must be either body floating (BF) or cardiac floating (CF) rated.
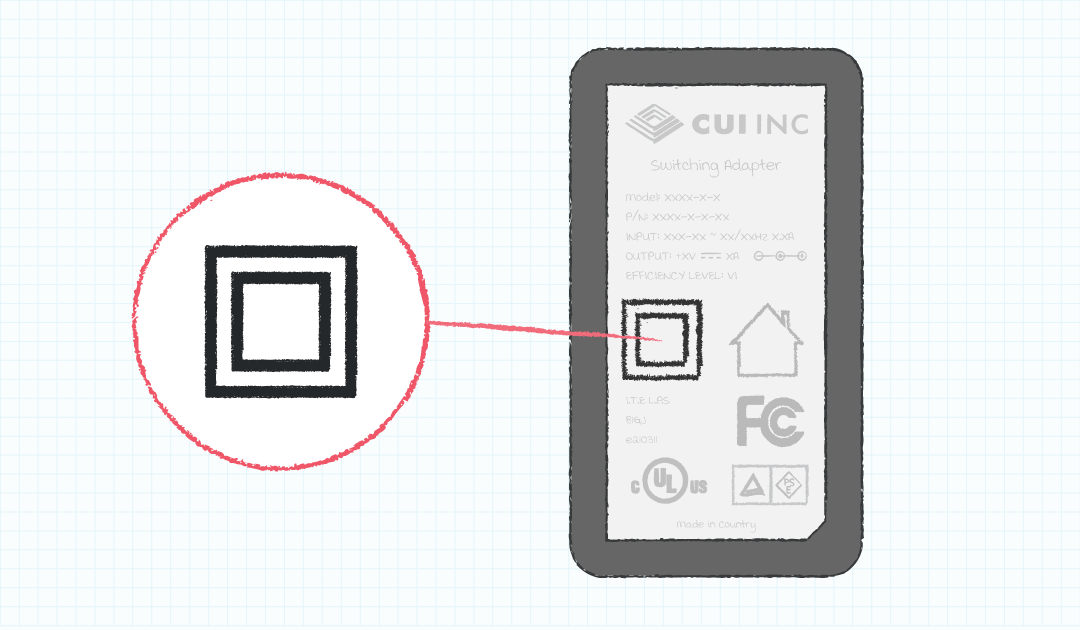
Class I equipment relies on the earth connection as a means of protection, which would be problematic if the home the equipment is used in doesn’t have proper earthing. Class II equipment uses double or reinforced insulation as its means of protection to eliminate the dependence on a properly installed earth connection.
Importance of Class B EMC for Home Healthcare Equipment
Electromagnetic compatibility (EMC) requirements are intended to ensure that equipment can properly function in the intended electromagnetic environment and will not interfere with other equipment in the area. Equipment is typically classified as Class A or B, where class B is more stringent and for a residential environment, and Class A applies to most other locations. The requirements for each class are defined in CISPR 11. Home healthcare equipment must be classified as Class B as it is intended for residential environments.
Shock and Vibration Testing
Home healthcare equipment must also be able to withstand a variety of shocks and vibration. Several equipment types are classified by IEC 6060-1-11 to account for different use cases and applications. These include fixed or stationary, hand-held, body-worn, portable, and mobile classifications for both non-transit operable and transit operable equipment.
Each case has its own requirements and must verify that basic safety and essential performance are maintained. Shock testing is defined in IEC 60068-2-27 and vibration in IEC 60068-2-64. Portable and handheld equipment must also undergo free fall tests according to IEC 60068-2-31. The type and magnitude of the tests depends on the equipment classification.
Environmental Concerns
Running too hot or too cold can have a big impact on a power supply. At cold temperatures, power supplies may have trouble starting up and could have trouble with ripple and regulation. Hot temperatures can trigger over temperature protections or lead to failure. While the environment in clinical environments is well controlled, homes may vary widely. The home healthcare standard specifies limits for both operating and storage/transport conditions (Table 1).
| Condition | Temperature | Humidity |
|---|---|---|
| Transport and Storage | -25°C to 70°C | 0% to 93% |
| Operation | 5°C to 40°C | 15% to 93% |
Transit operable equipment must also undergo testing for thermal shock. Thermal shock tests ramp the conditions from minimum to maximum over 5 minutes and then hold for 2 hours while essential performance is tested. The test is then reversed, ramping down from maximum to minimum and holding again for essential performance evaluation.
Power Supply Selection for Home Healthcare Applications
Power Supply companies, like CUI Inc, offer a broad range of internal and external power supplies that are medically rated. For home healthcare applications, care must be taken to verify that power supplies meet the additional requirements that IEC 60601-1-11 places on medical equipment. A solid understanding of the standard will help determine the proper equipment classifications and tests that are required to demonstrate compliance. CUI’s experienced team of power supply experts can help navigate the broad range of products and identify optimal solutions for your home healthcare application.
Product Selection , Safety & Compliance
You May Also Like
Have comments regarding this post or topics that you would like to see us cover in the future?
Send us an email at powerblog@cui.com