TELEHEALTH Keeping it Safe

The COVID-19 pandemic has been instrumental in changing the healthcare industry. Driving massive changes in an industry that has previously been much slower to adopt technological change due to the stringent standards that must be adhered to. One of the most significant developments is acceleration towards healthcare in the home.
A $250 BILLION MARKET
As we have all reduced our contact with other people, there has been huge growth in remote appointments via video or audio calls. Providers are seeing 50 to 175 times as many patients via telehealth than previously, as well as increases in home monitoring.
This trend to home healthcare was already happening over multiple years, of course, but has now shifted to overdrive. Demographic changes in many countries mean there is a higher proportion of elderly people, with more chronic medical issues that need management. There is pressure to keep the resulting growth in costs under control, which can be helped by patients and family members using equipment themselves.
At the same time, advances in technology mean that more is now possible – in terms of the medical technology itself, and the networking and wireless infrastructure needed to connect it all and send the data back to the professionals.
As this telehealth or virtual care industry takes off, consumers will most likely become used to the advantages it brings, in terms of convenience, timesaving, and access to care. McKinsey estimates the annual revenues of US telehealth vendors, pre-pandemic, was around US$3 billion – but the potential market size in the US is up to $250 billion.
This rapid growth raises new issues and challenges. For a start, we need medical technology that can be used by untrained patients, their family, or caregivers, particularly as recruiting more trained medical personnel is an expensive, long-term commitment. Without support staff around, the equipment needs to be reliable and dependable, and simple to operate. Data captured must be kept secure, to maintain patient confidentiality, and must be accurate, to avoid misdiagnosis.
Perhaps most importantly, we must be sure that everything brought into the home is safe – and that its safety can be verified.
WHAT ARE HOME MEDICAL DEVICES?
Fundamentally, products for use in the home need to meet higher standards to ensure safety is maintained. But there is a lack of consensus on what exactly should be included in this definition.
Arguably, remote appointments can be considered differently from other home usages because there will be a health professional present on the call . There is also room for interpretation as to what exactly is considered a medical device, as opposed to just a conventional technology product – a blood pressure monitor is on the list, but would you count a pulse monitor? Does it make a difference whether a product is branded as being health-related, or a fitness tracker, or a health-related mobile phone app (a huge and growing market)?
For this article, we’ll take a broad view and consider any medical-related item that may be used in the home or anywhere else outside of a conventional healthcare environment. It is always worth checking whether a particular product may not be subject to medical regulations, which will likely vary from country to country.
There are many examples, but some typical home medical products include:
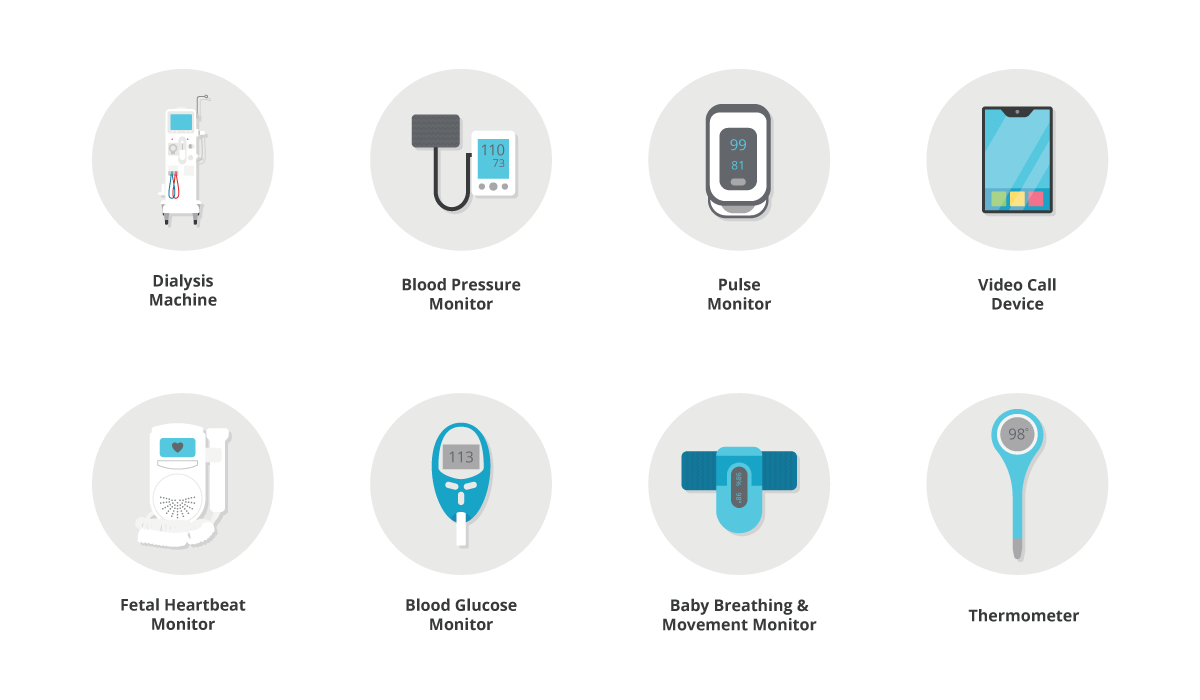
While some of these devices are simple and familiar, others can be complicated for patients and family members to come to grips with. People may also be dealing with multiple other issues, such as managing childcare or adapting to a new chronic condition, so we need to provide solutions that work the first time when they are needed most.
UNTRAINED USERS
When we’re looking at the safety of home healthcare devices, two key areas are most important to consider:
- Use by non-expert operators/patients
- Use in an uncontrolled environment
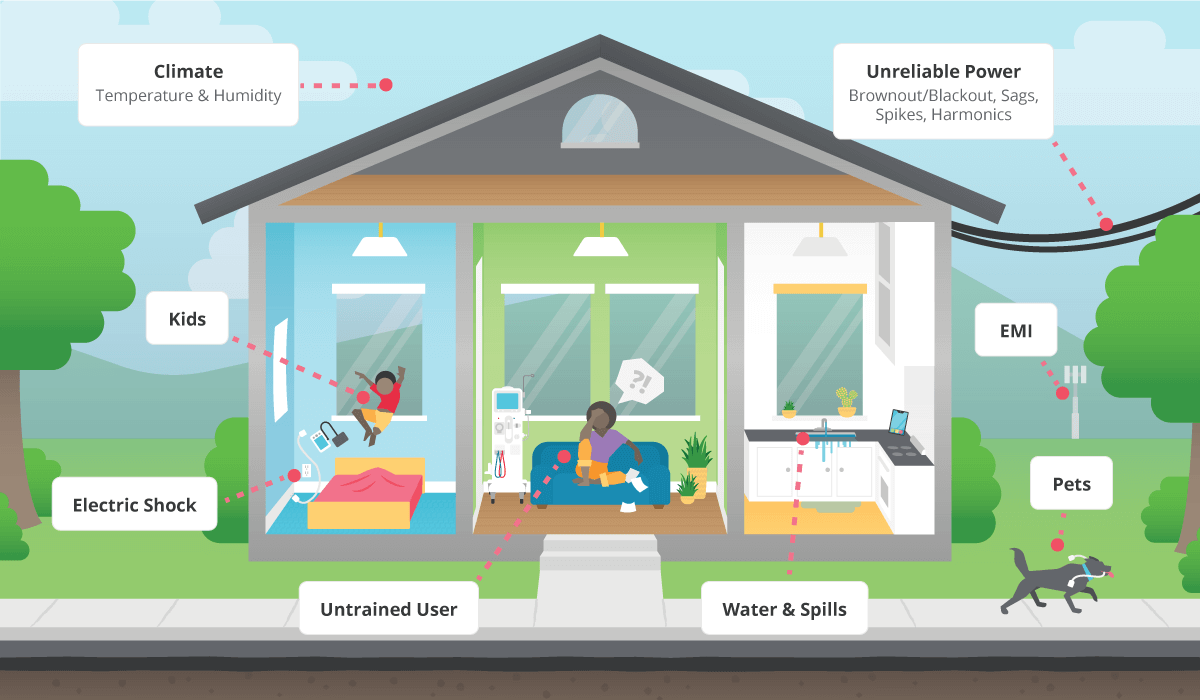
Without a trained operator, clear labelling and an easy-to-understand instruction manual are a must. The manual needs to be kept with the equipment so it isn’t lost, and it must provide sufficient detail without overwhelming the reader with jargon – research has shown that documentation is often designed for professionals, not patients. As well as written guides, online videos can be invaluable to make usage clear.
Translations to multiple languages must be considered, and whether non-native speakers will interpret everything clearly. Users also need a way to get support, whether that’s a phone helpline or an online presence. Training may also need to be considered, particularly if the user needs to calibrate or maintain the device, and even just to keep it clean.
Product designers need to consider any unconscious bias they may introduce. For example, will the device take into account the varying health differences among genders, like core temperature and hormone levels? Are there any issues around age or other demographic factors that have not been properly considered?
In terms of the device itself, it will need to be more robust for home use, as it is probably more likely to be dropped or to have food or drink spilled on it. Young children and pets can pose risks to delicate equipment. Also, a home user is perhaps less likely to report any potential damage than a professional.
To keep untrained users safe, the most important factor to consider is protection against electric shock. This can be helped by providing a higher level of isolation, and minimizing risks – for example, by keeping leakage current low.
Specifically, it is best to use Class II devices, so no single failure can leave a dangerous voltage exposed that could cause a shock. With this level of insulation, a product can be used safely without connection to suitable earth or ground.
UNCONTROLLED ENVIRONMENT
In the home, there are likely to be wide variations from the kind of environment you can expect in a hospital. For a start, the mains electrical supply may be unreliable, with insufficient grounding, and unpredictable dips and spikes in voltage. As mentioned above, a Class II product is usually required to ensure safe operation without a ground connection.
Electromagnetic compatibility (EMC) is another area to consider, and medical equipment for use in the home must be immune to problems from electromagnetic disturbance from other items, as well as keeping its emissions within appropriate limits.
The product designer needs to consider the impact of possible interruptions to the mains power supply. If this is serious, and if it endangers a patient’s health, then they need to provide a suitable Uninterruptible Power Supply (UPS) or backup power source, such as a generator.
Noise and sound levels are also unpredictable, which can be an issue if the medical device needs to make the user aware of an alarm. This is particularly relevant for patients with hearing impairments, as well as older people who generally have a much-reduced ability to hear high frequency sounds. Similarly, displays and screens need to be designed to work in varying light levels, and to be readable by people with sight problems.
CERTIFICATION AND REGULATION
The main international standard that addresses general safety and effectiveness issues for medical devices is IEC 60601-1, Medical electrical equipment – Part I: General requirements for basic safety and essential performance, which was last updated in 2015. As well as this general standard, there are multiple, more specific standards that provide more detail on particular products or usage.
For home healthcare, IEC 60601-1-11 provides particular requirements, which apply regardless of whether the equipment is expected to be used by the public, or by a trained operator.
IEC 60601-1-11 includes specific guidelines on usability that must be considered, to ensure untrained patients and operators can use a product safely. This includes avoiding confusion in controls and operating modes, and taking into account the potential for equipment to be disconnected.
There are some local variations, for example in the US a nursing home is considered a professional environment, so Class I protection is acceptable, while in Europe it is classified as a home environment that needs Class II . There are also varying levels of protection required depending on how the device is used, and specifically whether it comes into physical contact with the patient and an operator.
Table 1 below shows the different requirements for isolation, creepage, and insulation in IEC 60601-1 – including whether the requirement is for “Means of Operator Protection” (MOOP) or the stricter “Means of Patient Protection” (MOPP). The standard defines which classification is required in various usage scenarios: for example, equipment that makes physical contact with the patient, such as a blood pressure monitor, will typically need to meet the requirements for both two MOOP and one MOPP.
| CLASSIFICATION | ISOLATION | CREEPAGE | INSULATION |
|---|---|---|---|
| One MOOP | 1500 Vac | 2.5 mm | Basic |
| Two MOOP | 3000 Vac | 5 mm | Double/Reinforced |
| One MOPP | 1500 Vac | 4 mm | Basic |
| Two MOPP | 4000 Vac | 8 mm | Double/Reinforced |
Certification to IEC 60601-1-11 is required for the end product, rather than any particular sub-system or component. In practice, it makes a lot of sense to specify a power supply that is already certified, simplifying the approvals process and saving time.
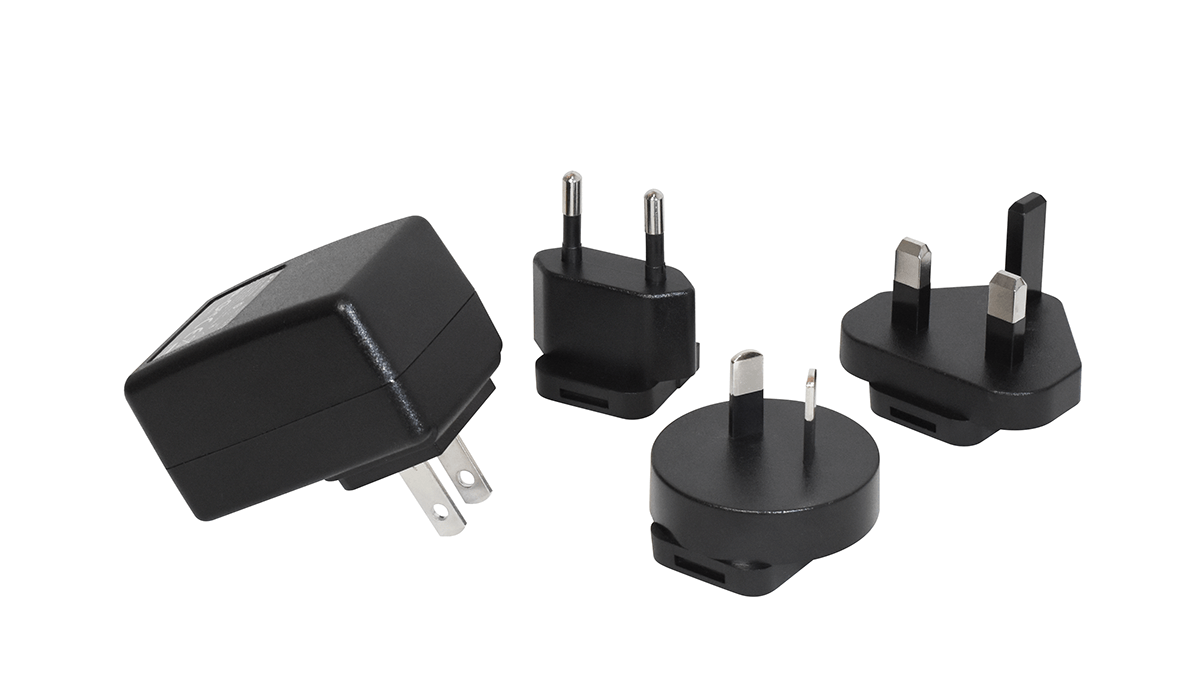
For instance, CUI’s medical power adapters are pre-approved for two MOPP applications. Figure 2 shows a typical example, the SMM6 series of 6W medical wall plug-in ac-dc power supplies.
CONCLUSION
While COVID-19 has focused attention on the growth of in-home healthcare, it is very much a long-term trend, driven by the needs of an aging population with increasing expectations. More people will be living with chronic conditions, needing monitoring, diagnosis, and treatment.
There is no doubt that telehealth can be highly effective, both in improving patient outcomes and saving money. For example, a project in the Netherlands provided daily remote monitoring and coaching for cardiac patients. This reduced hospital admissions by 52 percent and achieved high levels of patient and doctor satisfaction – while cutting costs by 26 percent.
In the near future, home medical devices are likely to become more capable and sophisticated, which in basic terms means more things can potentially fail. They may require multiple power supply rails for different components and keeping EMC issues under control may become more challenging.
Components need to be sourced when needed, including spare parts. CUI’s global network of distribution partners means it’s able to provide products quickly, from stock, at each stage of the design cycle to reduce delays.
For power supplies, complying with the relevant standards may involve some extra cost and complexity, but this can be minimized by choosing pre-certified power sub-systems – thus also reducing time to market.
Overall, the home healthcare market is an attractive, fast-growing opportunity for equipment vendors. While the safety requirements are stricter than for professional environments, with the help of suitable suppliers there is nothing that should prevent designers from developing healthcare products that can help people with their medical monitoring and treatments from the comfort of their own home.
